Johnson & Johnson said it is recalling about 574,000 bottles of its grape-flavored liquid infant Tylenol in the United States after parents complained about problems with the dosing system.
The recall is the latest in a long series for J&J’s consumer division involving Tylenol and other over-the-counter brands for adults and children.
No adverse events have been reported in association with Friday’s Tylenol recall, J&J said.
Copyright 2026 Reuters. Click for restrictions.
Was this article valuable?
Here are more articles you may enjoy.

 Judge Upholds $243M Verdict Against Tesla Over Fatal Autopilot Crash
Judge Upholds $243M Verdict Against Tesla Over Fatal Autopilot Crash  Besieged Berkshire Utility Tries to Rewrite Who Pays for Wildfires
Besieged Berkshire Utility Tries to Rewrite Who Pays for Wildfires 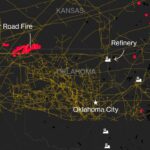 Explosive Wildfires Surge Through Oklahoma Panhandle and Kansas
Explosive Wildfires Surge Through Oklahoma Panhandle and Kansas  Walmart to Pay $100 Million to Settle FTC Case on Driver Wages
Walmart to Pay $100 Million to Settle FTC Case on Driver Wages 